My Research Projects
2024/05 - 2024/08: Sea Cucumber Collagen

Aww look how cute this guy is on the left! That is not the species im working on, the one im working on is so. ugly. (Cucumaria frondosa for those that are interested). Nathalie, the PhD student I work with, takes these lil guys and turns them into soluble collagen via a bunch of fun chemicals! (Don't worry they're already dead I promise) Then, I get to make things with said collagen! I've been working on a whole slew of applications from fibers to gels to adhesives, this guy's collagen is really versatile! I totally made the fibers by accident while trying to make gels but they're turning out to be really interesting mechanically.
My role in this project is to get the solubilized collagen, lyophilize it so it turns into a dry sponge, then redissolve it to get known concentration solutions. With these solutions I then get to play around and do whatever I want whether that be adding random chemicals, changing the pH, or drinking it (not actually). It's really cool because this collagen is sulphated, meaning it is anionic (negatively charged) rather than cationic (positively charged) as collagen typically is. This means we get an inverted solubility curve w.r.t. pH (yippee!!). Also the fibers are super easy to make but I lowkey cannot divulge how bc 'patent pending' or whatever. That being said, I also accidentally made an adhesive, so far it only works on polystyrene.
2023/05 - 2023/08: Blue Phase Liquid Crystals
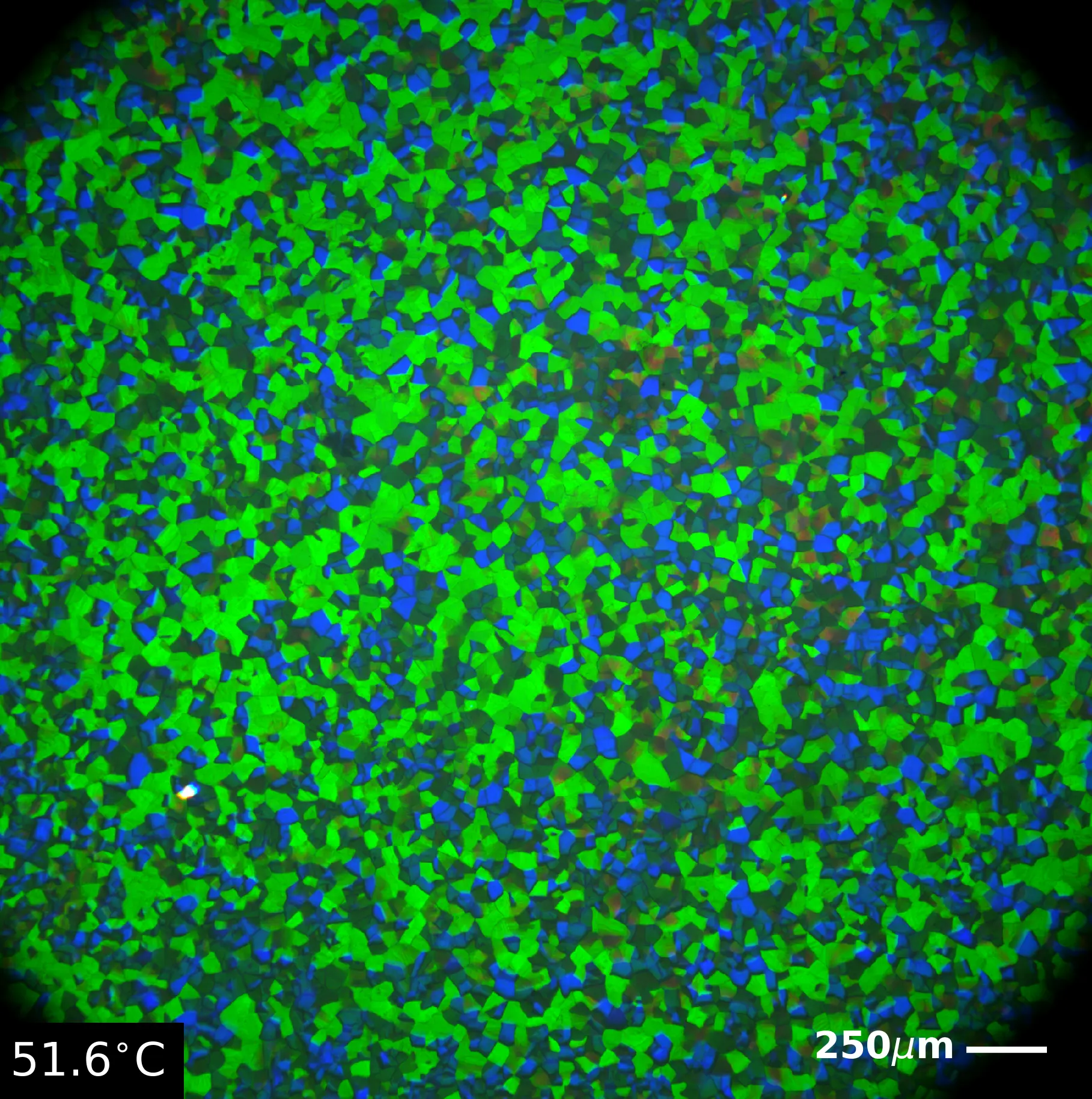
Woah beautiful mosaic! Imagine your job was to look at these all day every day (it got really boring I watched so much netflix in the microscope room). Still very pretty though. These funky lads are a special type of liquid crystal called a Blue Phase liquid crystal. Blue Phase II, pictured on the left, is ironically the one that appears first on a cooling cycle (I suppose the first time they saw them they were heating it up? Idk kind of a photosynthesis I and II moment). Blue phase liquid crystals can be made of a plethora of mixtures of polar LCs and are comprised of chiral helices of molecules arranged in a sort of lincoln-log esque way. This 3D structure gives them really cool properties like the potential for 3D laser emission!
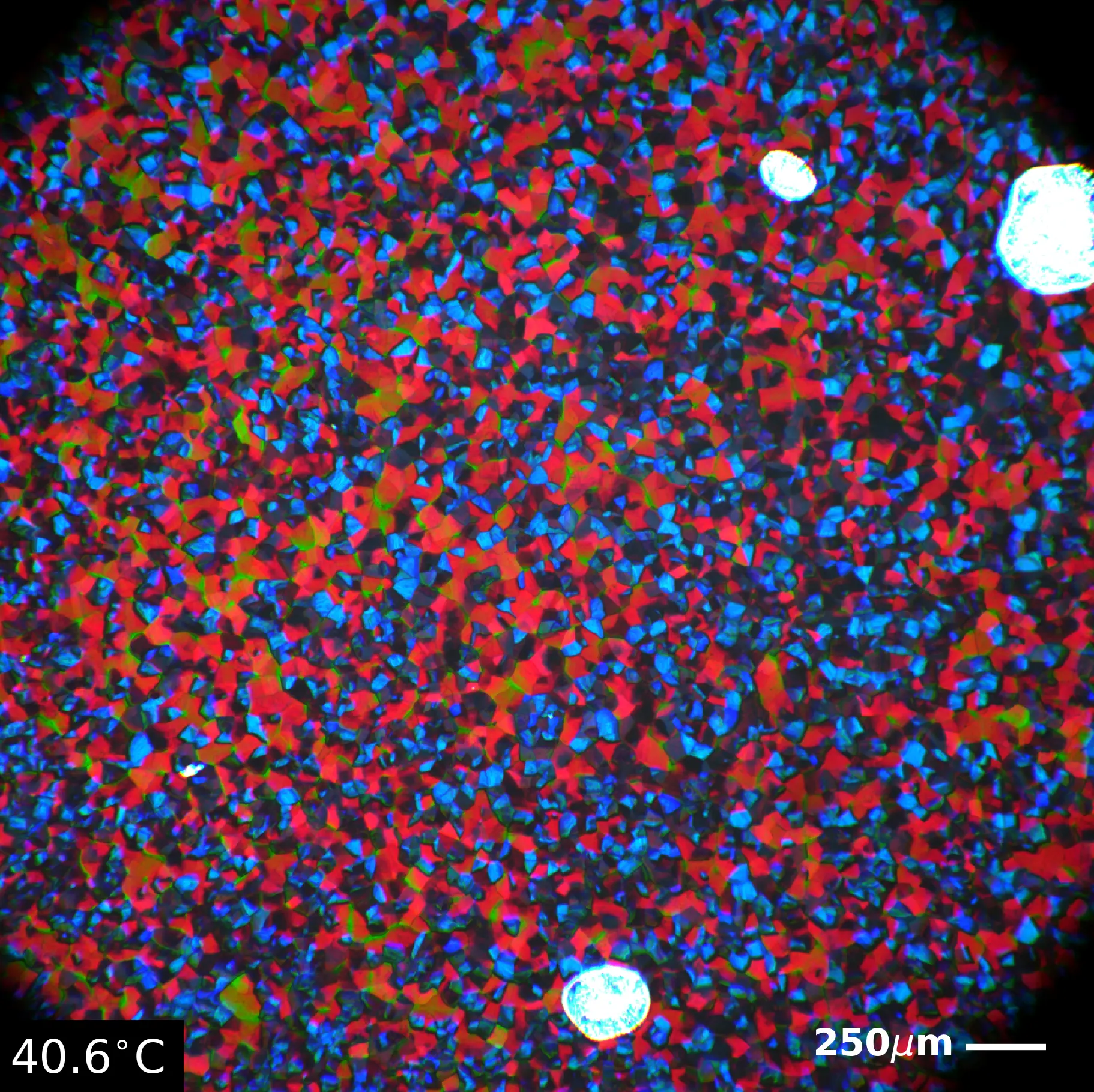 My job was to make these BPLC mixtures and then dope them with different amounts of different sizes of gold nanoparticles (AuNPs), isn't that so many words? I also had to make the AuNPs covered in 7-mEG rather than the DMAP or citrate they came in. The whole idea was that the AuNPs would fill the gaps in the lincoln log structure and make the BPLCs stable at room temp. I didn't end up succeeding but it did lower the stable window a little bit! The piccie on the right is BP I (ironically enough it's red, don't ask me scientists are weird) and it's super pretty! I really don't know how they came up with the name 'Blue Phase' because genuinely neither of these phases are majority blue. They're literally green and red. Maybe they were colorblind. Who knows.
My job was to make these BPLC mixtures and then dope them with different amounts of different sizes of gold nanoparticles (AuNPs), isn't that so many words? I also had to make the AuNPs covered in 7-mEG rather than the DMAP or citrate they came in. The whole idea was that the AuNPs would fill the gaps in the lincoln log structure and make the BPLCs stable at room temp. I didn't end up succeeding but it did lower the stable window a little bit! The piccie on the right is BP I (ironically enough it's red, don't ask me scientists are weird) and it's super pretty! I really don't know how they came up with the name 'Blue Phase' because genuinely neither of these phases are majority blue. They're literally green and red. Maybe they were colorblind. Who knows.
 Jessie
Jessie
My Papers
- Pb-Free Efficient Solar Cells
Review (PDF) - Nanoparticles in Drug Delivery
Mini-Review (PDF)
My Analysis Programs
Tensile Test Analysis
download .ipynb view .txtRaman Spectrum Analysis
download .ipynb view .txtSAXS/WAXS Analysis
download .ipynb view .txtZeta Potential Analysis
download .ipynb view .txt
Cool Literature
- I'm actually secretly illiterate